

2024-05-29
On May 29, 2024, BRL Medicine Inc., a company focused on gene and cell therapy, announced that the research results of the "Targeted CD19 Non-Viral PD1 Site-Specific Integration CAR-T Cell Injection" (Pipeline Code: BRL-201), developed based on its proprietary non-viral site-specific integration CAR-T platform for the treatment of relapsed/refractory B-cell non-Hodgkin lymphoma, have been successfully selected for the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. The latest clinical progress will be announced in a poster presentation format. This year's ASCO Annual Meeting will be held in Chicago, USA, from May 31st to June 4th local time. Notably, this is also the first time that BRL Medicine's BRL-201 research results have been publicly presented at an ASCO conference.

The research abstract of BRL-201 has been published on the official website of ASCO (Abstract number: 7031)
Regarding "BRL-201"
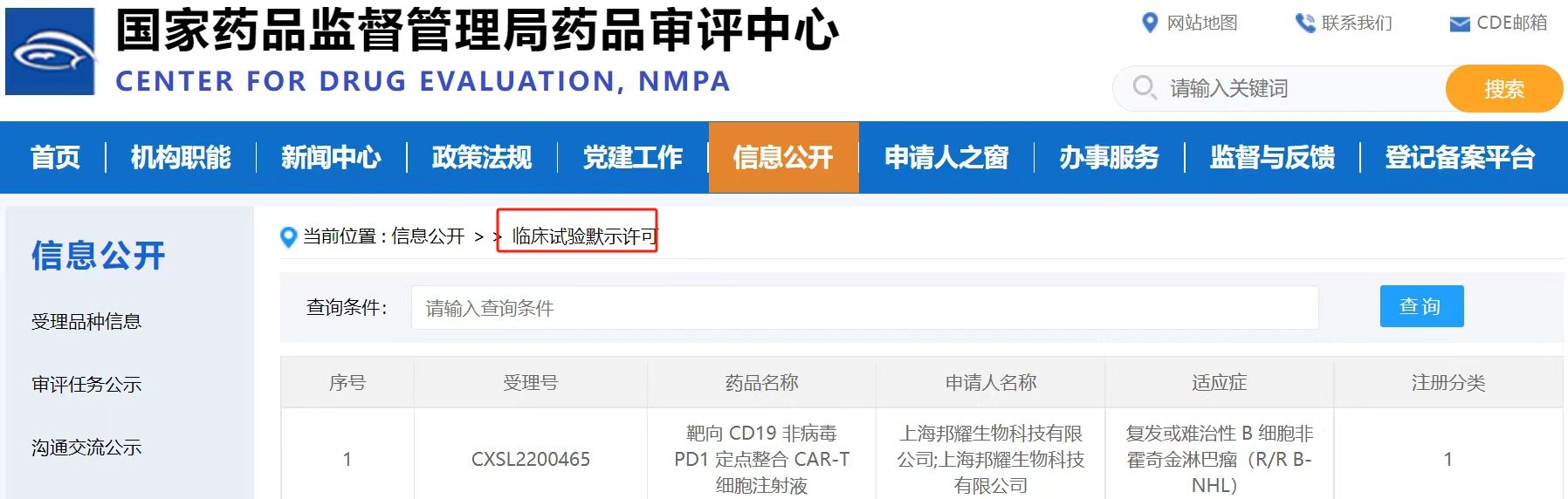
BRL-201 has been approved for China IND
On December 14, 2022,?BRL?Medicine?Inc. officially received approval?from the Center?for Drug Evaluation (CDE) of?the China Food?and Drug Administration?for the clinical trial application?(IND)?of?BRL-201, entering?the phase?of registered?clinical?trials.?This product?is developed using BRL?Medicine's proprietary non-viral targeted integration CAR-T platform?and is the world's first?targeted CD19 non-viral PD1-CAR-T product,?indicated for relapsed/refractory B-cell non-Hodgkin lymphoma?(R/R B-NHL). Non-Hodgkin lymphoma is a hematological malignancy originating in?the lymphoid tissue, accounting?for 80%-90%?of all?lymphomas. Although patients may achieve?remission after initial treatment, relapse?is common. Although CAR-T products have been approved for?the clinical treatment?of relapsed and refractory non-Hodgkin lymphoma, the overall?efficacy still needs to be?improved, and the toxic side effects caused by the massive cytokine release during treatment?also need to be reduced.
The ASCO poster session will?announce the latest clinical data of BRL-201?in?the IIT study.?The?research results show that?a total?of 21 patients received BRL-201 treatment,?with an objective?response?rate (ORR)?of up to 100%?and a complete response rate (CR)?of 85.7%. While achieving?significant efficacy, it did not cause cytokine release syndrome or neurotoxicity above grade 2?in any?patient. To date,?the first?patient in the world to receive?BRL-201 treatment?from BRL Medicine Inc. has?been cancer-free for more than 3 years.?This study demonstrates?the excellent clinical safety and efficacy?of BRL-201 product,?which can?be said?to be?the best clinical result?of high response rate and?low toxicity in?the global CAR-T cell therapy?for refractory and recurrent lymphoma. In addition, the research results?of?this product?were published in?the top international academic journal Nature on August 31, 2022.
In the future, BRL Medicine will continue to be patient-centered and work with clinical experts to fully promote the transformation and implementation of this clinical research achievement, bringing better treatment options for the majority of cancer patients.